Home
/ Nieuws / ...
Vitamine
D en de overlevingskans op de IC*
Uit
een Amerikaanse studie onder 932 mensen die op de Intensive Care werden
opgenomen blijkt dat goede bloedwaarden
vitamine D belangrijk zijn om te herstellen en de kans op doodgaan flink te
verminderen. Bij opname bleek 16,9% van de deelnemers een flink tekort (< 20
ng/ml) en 77,8% een tekort (< 30 ng/ml) te hebben, slechts 5,3% had goede
bloedwaarden vitamine D. Deelnemers met lage bloedwaarden vitamine D bleken ook
een fors hogere kans te hebben om dood te gaan
Vitamin
D Deficiency is Associated With Mortality in the Medical Intensive Care Unit
Sindhaghatta Venkatram; Sridhar Chilimuri;
Muhammad Adrish; Abayomi Salako; Madanmohan Patel; Gilda Diaz-Fuentes
Posted: 04/06/2012; Crit Care. 2011;15(6):R292 © 2011 BioMed
Central, Ltd.
Abstract
and Introduction
Abstract
Introduction:
The incidence of vitamin D deficiency in critically ill patients has been
reported to range from as low as 17% to as high as 79%. Data regarding the
relationship between 25-hydroxyvitamin D levels and outcomes in the medical
intensive care unit are sparse. The goal of the study was to evaluate the
prevalence of 25-hydroxyvitamin D deficiency in the medical intensive care unit
and its relationship with outcomes.
Method: This was a retrospective study in a medical intensive care unit
(MICU) at an inner city community hospital. The study period was between
October 2009 and February 2010.
Results: Of the 932 patients admitted during the study period,
25-hydroxyvitamin D vitamin D (25(OH)D) levels were available in 523 (53%); 86
of them were excluded from the study due to readmission to the intensive care
unit. Deficiency was defined as 0 to 19.9 ng/dL 25(OH)D levels, insufficiency
as 20 to 29.9 ng/dL, and normal levels as ≥30 ng/dL. Of the 437 patients
studied, 25(OH)D deficiency was identified in 340 (77.8%), insufficiency in 74
(16.9%), and normal levels in 23 (5.3%) patients. Patients with 25(OH)D
deficiency/insufficiency were younger (P = 0.015), were male (P =
0.001), and had kidney disease (P = 0.017) and lower total serum
calcium levels (P = 0.003). Hospital mortality was higher in patients
with 25(OH)D deficiency (P = 0.01). No differences in ventilator days
or length of stay in the MICU were evident among the three groups. Analysis by
multiple logistic regression demonstrated that acute physiology and chronic
health evaluation (APACHE) IV score ((odds ratio (OR) 1.036; 95% confidence
interval (CI) 1.0241.048, P < 0.0001), ventilator requirement (OR
7.7; 95% CI 4.313.98, P < 0.0001), 25 (OH) D levels(OR 0.942;
95% CI 0.9420.904, P < 0.0005) and 25(OH) D deficiency (OR 8.7;
95% CI 1.0372.8, P < 0.0469) showed statistical significance.
There was no association between 25(OH)]D insufficiency and hospital mortality.
The mean 25(OH)D level of survivors (27.9 ± 9.7 ng/dL) was higher than for
non-survivors (9.7 ± 4.7 ng/dL; P < 0.0001).
Conclusions: The study results demonstrate an association between
25(OH)D deficiency and hospital mortality in MICU patients. A randomized
prospective study to evaluate the effect of vitamin D replacement therapy on
mortality is warranted.
Introduction
Vitamin D is a fat-soluble vitamin that
regulates calcium metabolism. Adequate levels of 25(OH)D, the storage form of
vitamin D, are dependent on cutaneous synthesis stimulated by ultraviolet
radiation and/or adequate dietary intake of fortified foods and nutritional
supplements. A deficiency in 25(OH)D is estimated to exist in 50% to 60% of the
older population in North America and worldwide.[1] Recent evidence
suggests that the role of vitamin D is broader than the regulation of calcium
metabolism. Vitamin D has been shown to have anti-inflammatory and
anti-proliferative properties, and its deficiency has been linked to all-cause
mortality and cardiovascular disease and cancer.[25] The
incidence of 25(OH)D deficiency in critically ill patients has been reported to
range from as low as 17% to 79%.[68] Data regarding the
relationship between 25(OH)D levels and outcome in the medical intensive care
unit (MICU) are sparse with new reports suggesting the relationship of
deficiency in 25(OH)D and an increase in mortality in the critically ill.[69]
Most experts agree that levels less than 20
ng/dL 25 (OH)D are considered deficient and levels between 20 to 30 ng/dL are
insufficient.[1012]
The goal of the study was to evaluate the
prevalence of 25(OH)D deficiency in an inner-city MICU. The primary outcome was
hospital mortality, and secondary outcomes included duration of mechanical
ventilation and MICU length of stay. A subgroup analysis for primary and
secondary outcomes was performed for patients admitted from skilled nursing
facilities.
Materials
and Methods
Study
Design and Setting
This was a retrospective study of all
patients admitted to the MICU between October 2009 and February 2010 at a
26-bed closed unit. Our MICU is a university-affiliated inner-city hospital
staffed daily by two pulmonary and critical care-trained attending physicians,
a pulmonary fellow, and internal medicine residents.
Methods
All patients admitted to the MICU who had
levels of 25 (OH)D available were included in the study. Patients readmitted to
MICU during the same period of hospitalization were excluded, those patients
either already had 25(OH)D levels available from the first MICU admission and
repeated levels were not performed within 24 hours of MICU readmission or did
not any level available. The data were collected as part of a performance
improvement project looking to the prevalence of 25(OH)D deficiency in our MICU.
25(OH)D levels were collected randomly during the first 24 hours of admission
to the intensive care unit. There were no strict criteria to obtain 25(OH)D
levels. The physicians received education regarding the high prevalence of
hypovitaminosis in our community and were encouraged to evaluate for
hypovitaminosis when patients were admitted to the hospital. Baseline
demographics (age, gender, and race), history of end stage renal disease (ESRD)
or chronic kidney disease (CKD), as well as acute physiology and chronic health
evaluation (APACHE) IV were collected. The APACHE derived risk of death during
hospitalization was determined from the worst values obtained within 24 hours
of MICU admission. Clinical and laboratory variables obtained during the first
24 hours of hospital admission included serum levels of total calcium,
phosphate, creatinine, glucose, albumin and 25 (OH)D. Utilization and duration
of invasive mechanical ventilation, ICU length of stay (LOS), and hospital
mortality were analyzed. Length of stay in the MICU was defined as the time
from ICU admission to time of transfer out of the MICU. This study was approved
by the hospital institutional research review board, and the need for informed
consent was waived.
Serum 25(OH)D concentrations were assayed
by liquid chromatography-tandem mass spectrometry at Quest Diagnostics, New
York, NY. The analytical sensitivity is 4 ng/mL for 25OHD2 and 25OHD3
with a reportable range of 4 to 512 ng/mL for 25OHD2 and 25OHD3
Definition
of Vitamin D Deficiency
There is no firm consensus regarding
optimal levels of 25(OH)D. According to the workshop consensus conference for
vitamin D nutritional guidelines and a study that investigated the potential
beneficial effects of vitamin D for multiple health outcomes, the minimum
desirable serum level of 25(OH)D is suggested to be 20 to 30 ng/dL.[1,10,12]
Studies examining 25(OH)D deficiency in intensive care units have no agreement
on the cutoff levels for critically ill patients, with deficiency being defined
as 25(OH)D levels of less than 15 ng/dL to less than 29 ng/dL.[8,9,13]
In our study, we used a 25(OH)D cutoff level of less than 19.9 ng/dL to define
25(OH)D deficiency. 25(OH)D insufficiency was defined as 20 to 29.9 ng/dL.[11,14,15]
Definition
of Renal Failure
There is no consensus on the amount of
dysfunction that defines acute kidney injury, with more than 30 definitions in
use today.[16] Acute renal failure was defined as a serum creatinine
Χ >1.5 and acute or chronic renal failure as a worsening of renal function
in a patient with chronic kidney disease (serum creatinine Χ 3). End-stage
renal disease (ESRD) was defined as any patient with chronic kidney disease on
long term hemodialysis.[17]
Statistical
Analysis
Data analysis was conducted using the SPSS
v15.0. Discrete variables are expressed as counts (percentage) and continuous
variables as means ± standard deviations (SD). For the demographic and
clinical characteristics of the patients, differences between groups were
assessed using the chi-squared test and Fisher's exact test for categorical
variables and the Student's t-test or Mann-Whitney U test for continuous
variables. A one-way analysis of variance (ANOVA) was performed to explore the
impact of admitting diagnosis which were classified in nine different
categories: (a) Cardiac, (b) Gastrointestinal, (c) Metabolic, (d) Neurologic,
(e) Obstructive Airway Disease, (f) others, (g) Pulmonary, (h) Renal, and (i)
Sepsis, on the continuous dependent variable outcome of log-Vitamin D levels. A
multiple logistic regression model was performed for the whole population, with
mortality as the dependent variable, and age, gender, APACHE IV score,
ventilator requirement, acute/acute on chronic kidney disease, ESRD, serum
levels of total calcium, phosphate, creatinine, and 25(OH)D deficiency and
insufficiency as independent variables. Additionally, logistic regression with
a dependent variable of mortality was performed with a total of 10 independent
continuous variables; (a) APACHE, (b) age, (c) 25(OH)D levels, (d) ventilator
days, (e) ICU length of stay, (f) total calcium, (g) phosphate, (h) serum
creatinine, (i) serum albumin, and (j) serum glucose. A P value less
than 0.05 was considered statistically significant. To evaluate the prognostic
utility of 25(OH)D levels, a receiver-operating characteristic (ROC) curve was
constructed.
Results
A total of 932 patients were admitted to
the MICU during the 4-month study period. 25(OH)D levels were available for 523
(56%) patients; of these, 86 patients were excluded due to readmission to the
MICU during the same hospitalization. Of the 437 patients studied, 25(OH)D
deficiency was identified in 340 (77.8%) patients, insufficiency in 74 (16.9%)
patients, and normal levels in 23 (5.3%) patients (Figure 1). Characteristics
of the patients were stratified according to 25 (OH)D levels on admission (Table
1). Patients with 25 (OH)D deficiency/insufficiency were more likely to be
younger (P = 0.015), to be male (P = 0.001), to have acute/acute on CKD (P =
0.017), and to have lower total serum calcium levels (P = 0.003). A comparison
among the three 25(OH)D groups by admission diagnosis is shown in Table 2.
There were no differences in the mean log-25(OH)D levels among the nine
categories for admitting diagnosis. (P = .099). Comparison of 25-hydroxyvitamin
D levels among different admission diagnosis groups did not show statistical
significance (Table 3).
Table
1. Clinical characteristics of patients with available 25-hydroxyvitamin D
levels
|
Variable
|
25(OH)D
Deficiency ≤ 19.9 ng/dL N = 340
|
25(OH)D
Insufficiency 2029.9 ng/dL N= 74
|
25(OH)D
Normal ≥30 ng/dL N= 23
|
P
|
|
Age
(years)
|
55.6
± 16.5
|
58.6
± 19.2
|
65.4
± 16.6
|
0.015
|
|
Gender
- Male (%)
|
176
(51.8%)
|
28
(37.9%)
|
4
(17.4%)
|
0.001
|
|
APACHE
IV
|
68.3
± 28.1
|
60.9
± 25.9
|
67.2
± 20.9
|
0.11
|
|
Ventilator
requirement
|
123
(36.2%)
|
23
(31%)
|
7
(30.4%)
|
0.63
|
|
Acute/acute
on chronic renal failure
|
92
(27%)
|
11
(14.9%)
|
2
(8.7%)
|
0.017
|
|
ESRD
on hemodialysis
|
21
(7.1%)
|
6
(8.1%)
|
2
(8.7%)
|
0.92
|
|
Total
calcium serum (mg/dL)
|
8.6
± 1.1
|
9.0
± 1.1
|
9.4
± 1.3
|
0.0003
|
|
Phosphate
serum (mg/dL)
|
3.7
± 1.7
|
4.1
± 5.7
|
3.3
± 1.2
|
0.34
|
APACHE IV, acute physiology and chronic
health evaluation; ESRD, end-stage renal disease; N, Number; 25(OH)D,
25-hydroxyvitamin D
Table
2. Comparison of admission diagnosis groups based on deficient,
insufficient, and normal 25-hydroxyvitamin D levels
|
Variable
|
25(OH)D
Deficiency ≤ 019.9 ng/dL N (%) = 340
|
25(OH)D
Insufficiency 2029.9 ng/dL N (%) = 74
|
25(OH)D
Normal ≥ 30 ng/dL N (%) = 23
|
Total
N (%)
|
|
Cardiac
|
14(4%)
|
5(6.7%)
|
2(8.6%)
|
21(4.8%)
|
|
Gastrointestinal
|
39(11.4%)
|
5(6.7%)
|
3(13%)
|
47(10.7%)
|
|
Metabolic
|
39(11.4%)
|
8(10.8%)
|
3(13%)
|
50(11.4%)
|
|
Neurological
|
46(13.5%)
|
6(8.1%)
|
3(13%)
|
55(12.5%)
|
|
Obstructive
airway disease
|
42(12.3%)
|
17(22.9%)
|
1(4.3%)
|
60(13.7%)
|
|
Pulmonary
|
64(18.8%)
|
13(17.5%)
|
3(13%)
|
80(18.3%)
|
|
Others
|
34(10%)
|
10(13.5%)
|
4(17.3%)
|
48(10.9%)
|
|
Renal
|
19(5.5%)
|
3(4%)
|
0(0%)
|
22(5%)
|
|
Sepsis/Septic
shock
|
43(12.6%)
|
7(9.4%)
|
4(17.3%)
|
54(12.3%)
|
Table
3. Comparison of 25-hydroxyvitamin D levels between admission diagnosis
groups*
|
Admission
Diagnosis groups
|
Mean
25-hydroxyvitamin D (ng/dL) ± SD (range)
|
|
Cardiac
disorders. N (%)
|
15.1
± 11.1 (443)
|
|
Gastrointestinal
disorders. N (%)
|
12.5
± 10.1 (450)
|
|
Metabolic
disorders. N (%)
|
13.9
± 8.9 (440)
|
|
Neurological
disorders. N (%)
|
12.0
± 9.5 (451)
|
|
Obstructive
airway disease. N (%)
|
15.7
± 10.6 (474)
|
|
Pulmonary
disorders. N (%)
|
13.1
± 8.4 (442)
|
|
Others.
N (%)
|
16.6
± 11.7 (473)
|
|
Renal
disorders. N (%)
|
12.2
± 6.5 (426)
|
|
Sepsis/Septic
shock. N (%)
|
13.3
± 9.2 (442)
|
*No statistical significance was reached
between the groups
Comparisons of primary and secondary
outcomes are shown in Table 4.
Table
4. Comparison of outcomes based on deficient, insufficient, and normal
25-hydroxyvitamin D levels.
|
Variable
|
25(OH)D
Deficiency ≤019.9 ng/dL N = 340
|
25(OH)D
Insufficiency 2029.9 ng/dL N=74
|
25(OH)D
Normal ≥30 ng/dL N= 23
|
P
|
|
Actual
Hospital Mortality
|
82
(24.1%)
|
9
(12.2%)
|
1
(4.4%)
|
0.01
|
|
Predicted
ICU mortality [APACHE IV]
|
8.60%
|
7%
|
8%
|

|
|
Days
on a ventilator
|
6.9
± 6.0
|
5.9
± 6.0
|
6.4
± 5.1
|
0.77
|
|
ICU
length of stay [days]
|
4.3
± 4.5
|
3.7
± 3.9
|
4.2
± 3.7
|
0.54
|
APACHE IV, acute physiology and chronic
health evaluation; N, number; 25(OH)D, 25-hydroxyvitamin D.
Primary
Outcome
Hospital mortality was higher in patients
with 25(OH)D deficiency (P = 0.01). Comparisons of observed versus APACHE
IV-predicted mortality revealed that the observed mortality was higher than the
predicted mortality among patients with 25(OH)D deficiency (24.1% versus 8.6%)
and 25(OH)D insufficiency (12.2% versus 7%). However, in patients with normal
levels of 25(OH) D, the observed mortality was lower than predicted (4.4%
versus 8%).
Early (≤2 days) versus late mortality
is shown in Table 5. Classification percentages were similar between the
mortality and 25(OH)D groups, but a higher percentage of cases with
insufficient 25(OH)D experienced early mortality (13.8%) than late mortality
(7.9%). A higher percentage of cases with deficient 25(OH)D levels were
classified as late mortality (69.5%) versus early mortality (30.5%) (P
< 0.0005)
Table
5. Contingency table of cross tabulations between classifications of
Vitamin D levels and stages of mortality (N = 92)
|

|
25(OH)D
Deficiency ≤ 019.9 ng/dL N= 82
|
25(OH)D
Insufficiency 2029.9 ng/dL N=9
|
25(OH)D
Normal ≥30 ng/dL N=1
|
Total
|
|
Early
Mortality (frequency)
|
25
|
4
|
0
|
29
|
|
%
within mortality category
|
86.2
|
13.8
|
0.0
|
100.0
|
|
%
within Vitamin D category
|
30.5
|
44.4
|
0.0
|
31.5
|
|
%
of total
|
27.2
|
4.3
|
0.0
|
31.5
|
|
Late
Mortality (frequency)
|
57
|
5
|
1
|
63
|
|
%
within mortality category
|
90.5
|
7.9
|
1.6
|
100.0
|
|
%
within Vitamin D category
|
69.5
|
55.6
|
100.0
|
68.5
|
|
%
of total
|
62.0
|
5.4
|
1.1
|
68.5
|
N, number
Unadjusted and adjusted odds ratios (ORs)
for mortality are shown in Table 6. The following variables demonstrated
statistical significance after adjustment by multiple logistic regression
analysis: APACHE IV score (OR 1.036; 95% confidence interval (CI)
1.0241.048, P < 0.0001), ventilator requirement (OR 7.7; 95% CI
4.313.98, P < 0.0001), and 25(OH)D deficiency (OR 8.7; 95% CI
1.0372.8, P < 0.0469). No association between 25(OH)D
insufficiency and hospital mortality (OR 4.3; 95% CI 0.440.9, P =
0.2081) was evident.
Table
6. Logistic regression analysis for mortality risk using categorical
variables
|
Variable
|
Unadjusted
OR for Death
|
P
|
Adjusted
OR for Death
|
95%
CI
|
P
|
|
Ventilator
requirement
|
11.7
|
<0.0001
|
7.7
|
4.313.98
|
<0.0001
|
|
25(OH)D
deficiency
|
6.99
|
0.0289
|
8.7
|
1.0372.8
|
0.0469
|
|
25(OH)D
insufficiency
|
3.05
|
0.29
|
4.3
|
0.440.9
|
0.2081
|
APACHE IV, acute physiology and chronic
health evaluation; CI, confidence interval; 25(OH)D, 25-hydroxyvitamin D; OR,
odds ratio.
Logistic regression with a dependent variable of mortality and continuous
variables was performed for greater retention of information during analysis (Table
7). Wald statistics indicated that four variables contributed significantly to
the model: APACHE (χ2
= 29.01, OR 1.037; 95% CI 1.0231.050, P < .0005), 25(OH) D
levels (χ2 =
8.083, OR 0.94; 95% CI 0.9040.982, P = 0.004), admission albumin
levels (χ2
= 13.27, OR 0.457; 95% CI 0.0.3001.091, P < 0.0005), and
ventilator days (χ2
= 9.2, OR 1.15; 95% CI 1.0541.272, P = 0.002).
Table
7. Logistic regression analysis for mortality risk using continuous
variables
|
Variable
|
Wald
χ2
|
P-value
|
OR
|
95%
CI
|
|
APACHE
|
29.013
|
<.0005
|
1.037
|
1.0231.050
|
|
25(OH)D
|
8.083
|
.004
|
0.942
|
0.9040.982
|
|
Ventilator
days
|
9.285
|
.002
|
1.158
|
1.0541.272
|
|
Albumin
|
13.276
|
<.0005
|
0.457
|
0.3001.091
|
|
Age
|
0.432
|
.511
|
1.007
|
0.9871.027
|
|
ICU
LOS
|
0.015
|
.903
|
1.007
|
0.9061.118
|
|
Total
calcium
|
0.082
|
.775
|
0.963
|
0.7451.245
|
|
Phosphate
|
0.047
|
.828
|
1.012
|
0.9101.125
|
|
Creatinine
|
0.58
|
.446
|
0.946
|
0.8211.125
|
|
Glucose
|
1.778
|
.182
|
0.999
|
0.9970.696
|
APACHE IV, acute physiology and chronic
health evaluation; CI, confidence interval; 25(OH)D, 25-hydroxyvitamin D; LOS,
Length of stay; OR, odds ratio.
The mean 25(OH)D level for survivors (27.9
± 9.7 ng/dL) was higher than for non-survivors (9.7 ± 4.7 ng/dL; P
< 0.0001). The ROC curve for 25(OH)D levels is shown in Figure 2, and the
25(OH)D intersection curve is shown in Figure 3. The area under the curve (AUC)
was 0.66, and the cut-off value that maximizes sensitivity at 59.8% and
specificity at 58% is a 25(OH)D level of 10 ng/dL. Sensitivity and specificity
crossed at a probability level of 0.235 for the aforementioned 25(OH)D level.
With a prevalence rate of 77.8%, the positive predictive value (PPV) for
mortality with a 25(OH)D level less than 10 ng/dL was 83.64% and the negative
predictive value (NPV) was 29.52%.
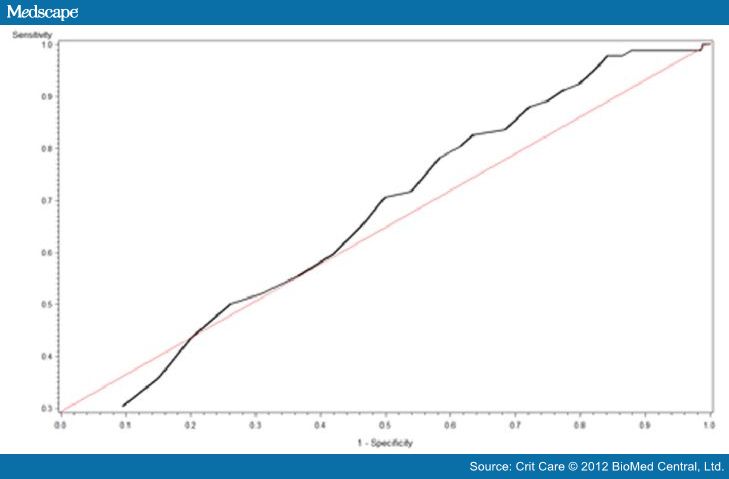
Figure
2. Vitamin D receiver
operating curve. Vitamin D receiver operating characteristic curve revealing an
area under the curve of 0.66.
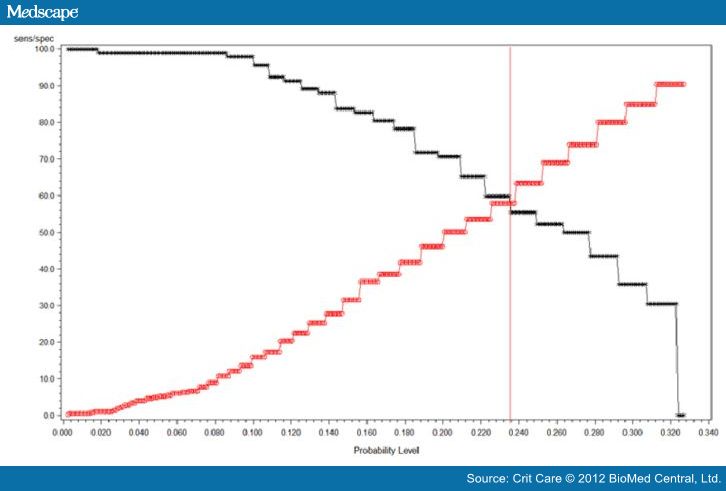
Figure
3. Vitamin D
intersection curve. Vitamin D intersection curve revealing sensitivity at 59.8%
and specificity at 58% for a vitamin D level of 10 ng/dL.
Secondary
Outcomes
Factors that did not significantly differ
between stratified groups were latitude, ventilator days and MICU length of
stay. The mean latitude for our study group was 40° north (SD 0.5° north).
There was no difference in ventilator days or MICU LOS for the different
admitting diagnosis groups.
Comparison of patients with available
25(OH)D levels versus patients without 25(OH)D revealed no difference for age,
gender, APACHE IV score, ventilator requirement or mortality (Figure 4).
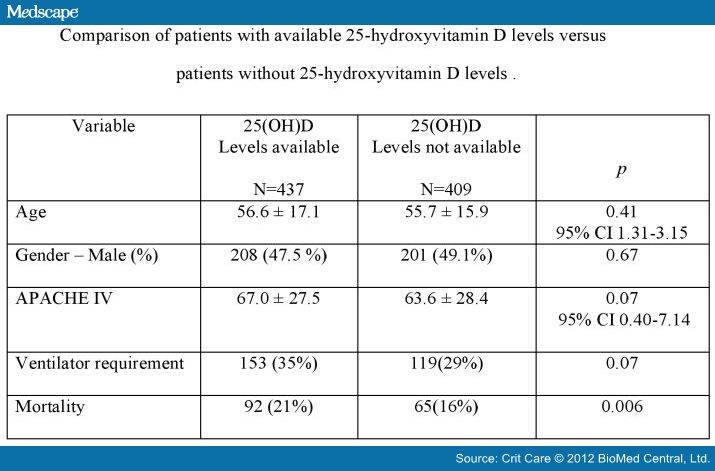
Figure
4. Comparison of
patients with available 25-hydroxyvitamin D levels versus patients without
25-hydroxyvitamin D levels.
Discussion
Our results demonstrate that 56% of
patients admitted to our MICU have a 25(OH)D deficiency. This incidence is
higher than other large studies showing a prevalence between 20% to 40%.[6,8,9]
Inner-city hospitals are unique in the sense that they care for an underserved
population with a higher rate of unemployment, lower income, higher use of
toxic substances and, in general, less than optimum medical care; all of which
lead to an increased incidence of uncontrolled diseases and a higher risk for
hypovitaminosis.
We found an association between 25(OH)D
deficiency and hospital mortality in our MICU population. 25(OH) D levels were
significantly higher in survivors than in non-survivors. In our study, total
serum hypocalcemia was not associated with an increased hospital mortality,
although levels of ionized calcium, 25(OH)D3 and PTH were not studied. Our data
are in line with another study showing a correlation between serum levels of
albumin and mortality.[5] Several explanations are possible for the
association between 25(OH)D deficiency and hospital mortality. The vitamin D
receptor is expressed in nearly all cells in the body, and the activating
enzyme 1-alpha-hydroxylase is expressed in many tissue types. Laboratory, cell
culture, and animal studies suggest that vitamin D may lower cancer risk by
inhibiting cell proliferation, angiogenesis, metastasis, and inflammation, as
well as inducing apoptosis and cellular differentiation. Several of these
mechanisms are relevant to atherosclerosis and cardiovascular disease, as well
as sepsis, respiratory failure, and other diseases commonly seen in the
critically ill.[12,1820] Deficiency of 25(OH) D has been
implicated as a cause of increased cardiovascular events and death.[2124]
The increased mortality in the critically ill with vitamin D deficiency might
be due to changes in glucose and calcium metabolism, and/or immune and
endothelial cell dysfunction due to the deficiency.[2529]
Endothelial cell dysfunction has been
proposed as a potential cause of multiple organ dysfunction syndrome.[3032]
It is possible that 25(OH)D deficiency amplifies the metabolic derangements and
impaired immune regulation seen in critically ill states, which may lead to
worse outcomes than would be experienced with normal vitamin D levels.
Furthermore, 25(OH)D deficiency has been implicated in sepsis, stroke,
inflammatory bowel disease, autoimmune conditions and asthma.[3339]
Contrary to the study by McKinney et al.,
we did not find a correlation between 25(OH)D deficiency and an increased
length of stay among patients admitted to the MICU; it is important to note
that in their study the LOS was dichotomized to a LOS less and more than three
days, respectively.[8]
Risk factors for low vitamin D levels
include older age, living in northern latitudes, sun avoidance, dark skin
pigmentation, obesity, low dietary intake of vitamin D, and various medical
conditions, especially malabsorption syndromes. These factors are especially
important for older patients in nursing home facilities.[40]
Causes of low 25(OH)D levels in patients
admitted to ICUs are multifactorial. In addition to the well-known etiologies,
it is important to consider other factors such as interaction with medications,
abnormal gastrointestinal function and the effect of fluid resuscitation.[41]
Contrary to our expectations and reports in
the literature, our study showed that patients with either 25(OH) D deficiency
or insufficiency were generally younger than patients with normal 25(OH)D
levels and they were predominantly of male gender. The association between
25(OH)D levels and hospital mortality in men and in younger patients is unclear.
Most published studies show a higher prevalence of vitamin D deficiency in
women and the elderly.[9,42,43] The large multicenter study done by
Braun et al. confirmed our association between low 25(OH)D levels and younger
age, but not with male gender.[9] These findings could be just a
reflection of the general vitamin deficiency in our population.
In our cohort, 93% of patients with ESRD
and 98% of patients with acute and acute on CKD had 25(OH)D deficiency/insufficiency,
and these findings are consistent with other published findings.[14,4449]
Chronic kidney disease is characterized by decreased renal phosphate excretion,
with resultant increases in serum phosphate levels; furthermore, there is
decreased conversion of vitamin D to its active form, 1,25-dihydroxyvitamin D3
(1,25(OH)D3), resulting in decreased levels of circulating 1,25(OH)D3 and serum
calcium and decreased intestinal calcium absorption. The hyperphosphatemia,
hypocalcemia, and decreased levels of active vitamin D result in increased
synthesis and secretion of parathyroid hormone. Some studies found no
interaction between low levels of 25(OH)D and PTH concentrations or calcium
levels. This could suggest that the association of 25(OH)D status and mortality
is not significantly modified by PTH or calcium levels.[6,47,50]
Vitamin D deficiency has been associated with cardiovascular mortality and
all-cause mortality in patients with CKD.[4447,5052] There is
no conclusive data regarding vitamin D supplementation and decrease in
mortality or other outcomes in critically ill patients. A meta-analysis of
randomized controlled trials suggested that supplementation of 400 to 830 IU of
vitamin D decreased mortality in the general population during the trial
periods.[21] In a subsequent study, there was no association between
vitamin D classes and mortality.[6] Levels of 25(OH)D ≥150 ng/dL
are potentially harmful and are associated with elevated risk of hypercalcemia,
vascular soft tissue calcification, and hyperphosphatemia.[53]
Vitamin D intoxication can potentially be life-threatening but the majority of
officially recorded cases could be related to prolonged intakes of >40,000
IU per day.[54] One small study looking at the short-term metabolic
effect of high dose oral vitamin D3 replacement in the intensive care unit did
not reveal any complications.[55] A recent Cochrane review of fifty
randomized trials with 94,148 participants showed that vitamin D in the form of
vitamin D3 seems to decrease mortality in predominantly elderly
women.[56]
Our work has several potential limitations.
First, this was a retrospective single center study and we did not sample
25(OH)D levels sequentially. The 25(OH)D levels obtained on admission are
probably a reflection of pre-admission deficiency. Vitamin D levels were not
available for all patients in this cohort; however, analysis of the groups with
and without available vitamin D levels reflected no gross bias. Second, the
study was completed in the fall and winter months, which have been
traditionally associated with lower levels of vitamin D, and may have
overestimated the deficiency of vitamin D in our population. Third, our study
was conducted in a MICU and cannot be generalized to cardiac, surgical, or
cardiothoracic units. Fourth, our study did not intend to evaluate the
association of low 25(OH)D levels and inflammatory markers or incidence of
infectious diseases, neither did we attempt to see the effect of 25 (OH)D
replacement on mortality. Finally, PTH and 1,25D3 levels were not available and
we cannot exclude the confounding effects of these variables.
Conclusions
In conclusion, we report on a large cohort
of patients with 25(OH)D deficiency and insufficiency in a MICU setting. Our
study shows a clear association between 25(OH)D levels and hospital mortality
in critically ill patients. 25(OH)D levels of 10 ng/dL predicted hospital
mortality in 83.6% of this cohort. The observed hospital mortality for 25(OH)D
deficient patients was higher than the predicted mortality based on admission
APACHE IV score.
The finding that 25(OH)D deficiency,
especially at levels less than 10 ng/dL, is associated with increased hospital
mortality has both clinical and research implications. Clinically, patients
admitted to the medical ICU who present with a 25(OH)D deficiency are at
greater risk for short-term hospital mortality and may therefore potentially
benefit from more intensive surveillance at ICU admission. Future studies are
needed to answer some of the most relevant questions such as: Is 25(OH) D
deficiency merely another marker for severity of illness? Can hospital
mortality, risk of infections, LOS in the intensive care unit be changed or
modulated just by evaluating and correcting 25(OH)D deficiency ? What are the
optimal doses for replacement and what is the long term outcome in those
patients? Future research is warranted to determine whether correction of
25(OH)D deficiency is associated with improved outcomes for ICU patients.
Sidebar
Key
Messages
- 25(OH)D
deficiency and insufficiency is a common finding in a medical intensive care
unit.
- 25(OH)D
deficiency in the intensive care unit is associated with increased risk for
hospital mortality.
- There
was correlation between 25(OH)D deficiency and late mortality (≥2 days)
whereas this effect was not seen in 25(OH)D insufficiency.
- A
25(OH)D level less than 10 ng/dL had a positive predictive value for
hospital mortality of 83.6%.
- Measurement
of 25(OH)D D levels should be considered as part of the routine initial
laboratory tests obtained in the medical intensive care unit.
References
- Norman
AW, Bouillon R, Whiting SJ, Veith R, Lips P: 13th Workshop Consensus for
Vitamin D Nutritional Guidelines. J Steroid Biochem Mol Biol
2007, 103:204205.
- Dobnig
H, Pilz S, Scharnagl H, Renner W, Seelhorst U, Wellnitz B, Kinkeldei J,
Boehm BO, Weihrauch G, Maerz W: Independent association of low serum
25-hydroxyvitamin D and 1,25-dihydroxyvitamin D levels with all-cause and
cardiovascular mortality. Arch Intern Med 2008, 168:13401349.
- Lee
JH, O'Keefe JH, Bell D, Hensrud DD, Holick MF: Vitamin D deficiency: an
important, common, and easily treatable cardiovascular risk factor? J Am
Coll Cardiol 2008, 52:19491956.
- Zittermann
A, Gummert JF, Bφrgermann J: Vitamin D deficiency and mortality. Curr
Opin Clin Nutr Metab Care 2009, 12:634639.
- Melamed
ML, Michos ED, Post W, Astor B: 25-hydroxyvitamin D levels and the risk of
mortality in the general population. Arch Intern Med 2008,
168:16291637.
- Lucidarme
O, Messai E, Mazzoni T, Arcade M, du Cheyron D: Incidence and risk factors
of vitamin D deficiency in critically ill patients: results from a
prospective observational study. Intensive Care Medicine 2010,
36:16091611.
- Lee
P, Eisman JA, Center JR: Vitamin D deficiency in critically ill patients. N
Engl J Med 2009, 360:19121914.
- McKinney
JD, Bailey BA, Garrett LH, Peiris P, Manning T, Peiris AN: Relationship
between vitamin D status and ICU outcomes in veterans. J Am Med Dir
Assoc 2011, 12:208211.
- Braun
A, Chang D, Mahadevappa K, Gibbons FK, Liu Y, Giovannucci E, Christopher KB:
Association of low serum 25-hydroxyvitamin D levels and mortality in the
critically ill. Crit Care Med 2011, 39:671677.
- Bischoff-Ferrari
HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B: Estimation of
optimal serum concentrations of 25-hydroxyvitamin D for multiple health
outcomes. Am J Clin Nutr 2006, 84:1828, Review. Erratum in: Am J
Clin Nutr 2006, 84:1253.
- Malabanan
A, Veronikis IE, Holick MF: Redefining vitamin D insufficiency. Lancet
1998, 351:805806.
- Holick
MF: Vitamin D deficiency. N Engl J Med 2007, 357:266281.
- Lee
P, Eisman JA, Center JR: Vitamin D deficiency in critically ill patients. N
Engl J Med 2009, 360:19121914.
- Thomas
MK, Lloyd-Jones DM, Thadhani RI, Shaw AC, Deraska DJ, Kitch BT, Vamvakas EC,
Dick IM, Prince RL, Finkelstein JS: Hypovitaminosis D in medical inpatients.
N Engl J Med 1998, 338:777783.
- Holick
MF: High prevalence of vitamin D inadequacy and implications for health. Mayo
Clin Proc
2006, 81:353373.
- Kellum
JA, Levin N, Bouman C, Lameire N: Developing a consensus classification
system for acute renal failure. Curr Opin Crit Care 2002,
8:509514.
- Bellomo
R, Ronco C, Kellum JA, Mehta RL, Palevsky P: Acute renal failure
definition, outcome measures, animal models, fluid therapy and information
technology needs: The Second International Consensus Conference of the Acute
Dialysis Quality Initiative [ADQI] Group. Crit Care 2004,
8:R204-R212.
- Bouillon
R, Carmeliet G, Verlinden L, van Etten E, Verstuyf A, Luderer HF, Lieben L,
Mathieu C, Demay M: Vitamin D and human health: lessons from vitamin D
receptor null mice. Endocr Rev 2008, 29:726776.
- Veldman
CM, Cantorna MT, DeLuca HF: Expression of 1, 25-dihydroxyvitamin D [3]
receptor in the immune system. Arch Biochem Biophys 2000,
374:334338.
- Zittermann
A: Vitamin D and disease prevention with special reference to cardiovascular
disease. Prog Biophys Mol Biol 2006, 92:3948.
- Autier
P, Gandini S: Vitamin D supplementation and total mortality: a meta-analysis
of randomized controlled trials. Arch Intern Med 2007,
167:17301737.
- Kendrick
J, Targher G, Smits G, Chonchol M: 25 Hydroxyvitamin D deficiency is
independently associated with cardiovascular disease in the Third National
Health and Nutrition Examination Survey. Atherosclerosis 2009,
205:255260.
- Fiscella
K, Franks P: Vitamin D, race, and cardiovascular mortality: findings from a
national US sample. Ann Fam Med 2010, 8:1118.
- Giovannucci
E, Liu Y, Hollis BW, Rimm EB: 25-Hydroxyvitamin D and risk of myocardial
infarction in men: a prospective study. Arch Intern Med 2008,
168:11741180.
- Lee
P, Nair P, Eisman JA, Center JR: Vitamin D deficiency in the intensive care
unit: an invisible accomplice to morbidity and mortality? Intensive Care
Med 2009, 35:20282032.
- van
den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M,
Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R: Intensive insulin
therapy in critically ill patients. N Engl J Med 2001,
345:13591367.
- Zivin
JR, Gooley T, Zager RA, Ryan MJ: Hypocalcemia: a pervasive metabolic
abnormality in the critically ill. Am J Kidney Dis 2001,
37:689698.
- Desai
TK, Carlson RW, Geheb MA: Prevalence and clinical implications of
hypocalcemia in acutely ill patients in a medical intensive care setting. Am
J Med 1988, 84:209214.
- Burchard
KW, Gann DS, Colliton J, Forster J: Ionized calcium, parathormone, and
mortality in critically ill surgical patients. Ann Surg 1990,
212:543549.
- Aird
WC: Endothelium as an organ system. Crit Care Med 2004, 32:
S271279.
- Aird
WC: Endothelial cell dynamics and complexity theory. Crit Care Med
2002, 30:S180185.
- Aird
WC: The role of the endothelium in severe sepsis and the multiple organ
dysfunction syndrome. Blood 2003, 101:37653777.
- Jeng
L, Yamshchikov AV, Judd SE, Blumberg HM, Martin GS, Ziegler TR, Tangpricha
V: Alterations in vitamin D status and anti-microbial peptide levels in
patients in the intensive care unit with sepsis. J Transl Med 2009,
7:28.
- Pilz
S, Dobnig H, Fischer JE, Wellnitz B, Seelhorst U, Boehm BO, Mδrz W: Low
vitamin D levels predict stroke in patients referred to coronary angiography.
Stroke 2008, 39:26112613.
- Pappa
HM, Grand RJ, Gordon CM: Report on the vitamin D status of adult and
pediatric patients with inflammatory bowel disease and its significance for
bone health and disease. Inflamm Bowel Dis 2006,
12:11621174.
- Arnson
Y, Amital H, Shoenfeld Y: Vitamin D and autoimmunity: new aetiological and
therapeutic considerations. Ann Rheum Dis 2007,
66:11371142.
- Adorini
L, Penna G: Control of autoimmune diseases by the vitamin D endocrine system.
Nat Clin Pract Rheumatol 2008, 4:404412.
- Zold
E, Szodoray P, Gaal J, Kappelmayer J, Csathy L, Gyimesi E, Zeher M, Szegedi
G, Bodolay E: Vitamin D deficiency in undifferentiated connective tissue
disease. Arthritis Res Ther 2008, 10:R123.
- Sutherland
ER, Goleva E, Jackson LP, Stevens AD, Leung DY: Vitamin D levels, lung
function, and steroid response in adult asthma. Am J Respir Crit Care
Med 2010, 181:699704.
- Hirani
V, Primatesta P: Vitamin D concentrations among people aged 65 years and
over living in private households and institutions in England: population
survey. Age Ageing 2005, 34:485491.
- Krishnan
A, Ochola J, Mundy J, Jones M, Kruger P, Duncan E, Venkatesh B: Acute fluid
shifts influence the assessment of serum vitamin D status in critically ill
patients. Critical Care 2010, 14:R216.
- Sambrook
PN, Cameron ID, Cumming RG, Lord SR, Schwarz JM, Trube A, March LM: Vitamin
D deficiency is common in frail institutionalised older people in northern
Sydney. Med J Aust 2002, 176:560.
- Brock
K, Wilkinson M, Cook R, Lee S, Bermingham M: Associations with vitamin D
deficiency in "at risk" Australians. J Steroid Biochem
Mol Biol 2004, 8990:581588.
- Gonzαlez
EA, Sachdeva A, Oliver DA, Martin KJ: Vitamin D insufficiency and deficiency
in chronic kidney disease. A single center observational study. Am J
Nephrol 2004, 24:503510.
- Saab
G, Young DO, Gincherman Y, Giles K, Norwood K, Coyne DW: Prevalence of
vitamin D deficiency and the safety and effectiveness of monthly
ergocalciferol in hemodialysis patients. Nephron Clin Pract 2007,
105:c132-c138.
- Jean
G, Terrat JC, Vanel T, Hurot JM, Lorriaux C, Mayor B, Chazot C: Daily oral
25-hydroxycholecalciferol supplementation for vitamin D deficiency in
haemodialysis patients: effects on mineral metabolism and bone markers. Nephrol
Dial Transplant 2008, 23:36703676.
- Pilz
S, Tomaschitz A, Friedl A, Amrein K, Drechsler C, Ritz E, Boehm BO, Grammer
TB, Mδrz W: Vitamin D status and mortality in chronic kidney disease. Nephrol
Dial Transplant 2011, 26:36033609.
- Pecovnik-Balon
B, Jakopin E, Bevc S, Knehtl M, Gorenjak M: Vitamin D and mortality as a
novel nontraditional risk factor for mortality in hemodialysis patients. Ther
Apher Dial 2009, 13:268272.
- Drechsler
C, Pilz S, Obermayer-Pietsch B, Verduijn M, Tomaschitz A, Krane V, Espe K,
Dekker F, Brandenburg V, Mδrz W, Ritz E, Wanner C: Vitamin D deficiency is
associated with sudden cardiac death, combined cardiovascular events, and
mortality in haemodialysis patients. Eur Heart J 2010,
31:22532261.
- Ravani
P, Malberti F, Tripepi G, Pecchini P, Cutrupi S, Pizzini P, Mallamaci F,
Zoccali C: Vitamin D levels and patient outcome in chronic kidney disease. Kidney
Int 2009, 75:8895.
- Cheng
S, Coyne D: Vitamin D and outcomes in chronic kidney disease. Curr
Opin Nephrol Hypertens 2007, 16:7782.
- Mehrotra
R, Kermah DA, Salusky IB, Wolf MS, Thadhani RI, Chiu YW, Martins D, Adler SG,
Norris KC: Chronic kidney disease, hypovitaminosis D, and mortality in the
United States. Kidney Int 2009, 76:977983.
- Jones
G: Pharmacokinetics of vitamin D toxicity. Am J Clin Nutr 2008,
88:582S-586S.
- Vieth
R: Critique of the considerations for establishing the tolerable upper
intake level for vitamin D: critical need for revision upwards. J Nutr
2006, 136:11171122.
- Amrein
K, Sourij H, Wagner G, Holl A, Pieber TR, Smolle KH, Stojakovic T, Schnedl
C, Dobnig H: Short-term effects of high-dose oral vitamin D3 in critically
ill vitamin D deficient patients: a randomized, double-blind,
placebo-controlled pilot study. Critical Care 2011, 15:R104.
- Bjelakovic
G, Gluud LL, Nikolova D, Whitfield K, Wetterslev J, Simonetti RG, Bjelakovic
M, Gluud C: Vitamin D supplementation for prevention of mortality in adults.
Cochrane Database of Systematic Reviews 2011, , 7: CD007470, DOI:
10.1002/14651858.CD007470.pub2.
Abbreviations
ANOVA: one-way analysis of variance; APACHE: acute physiology and chronic
health evaluation; CKD: chronic kidney disease; ESRD: end stage renal disease;
ICU: intensive care unit; MICU: medical intensive care unit; 25(OH)D:
25-hydroxyvitamin D; OR: odds ratio; PTH: parathyroid hormone; ROC:
receiver-operating characteristic; SD: standard deviation.
Crit
Care. 2011;15(6):R292 © 2011 BioMed Central, Ltd.
Copyright to this article is held by the author(s), licensee BioMed Central Ltd.
This is an Open Access article: verbatim copying and redistribution of this
article are permitted in all media for any purpose, provided this notice is
preserved along with the article's original citation.
(Juni 2012)
Printen
Reacties:


